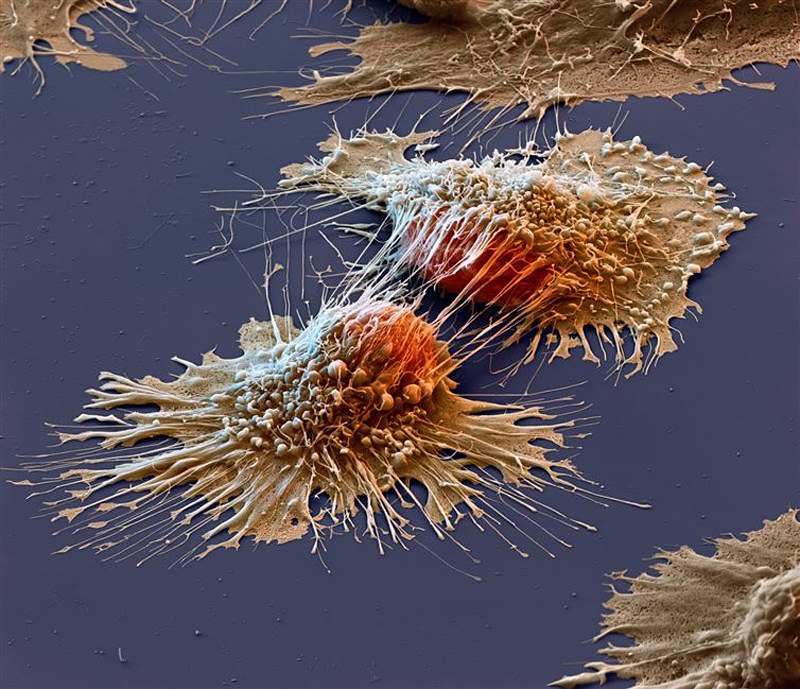
Theoretically, the more cells an organism has, the higher the incidence of malignant transformation. If this were true, humans should be considerably more cancer-prone than something as small as a mouse; however, this is not the case. “Peto’s paradox” describes the phenomenon that, despite the increase in cell number, instances of cancer don’t increase in larger, more complex animals. Researchers over the past several decades have determined that the complexity of organisms such as humans, elephants, and whales coincides with the evolution of more advanced tumor suppressor mechanisms.
Typically, in defense against cancer, the p53 gene induces an apoptotic response, effectively killing cancerous cells to prevent further carcinogenesis. P53 is present in high numbers in the elephant genome, answering Peto’s paradox. Consistent cell death can prevent cancer but does not necessarily promote longevity. The ability of bowhead whales, for example, to seldom experience cancer while also experiencing centuries of life is certainly catching the attention of researchers looking for more effective cancer prevention and treatment for humans.
The bowhead whale’s lifespan is longer than that of any other mammal, exceeding 200 years. The whale species is the second largest animal on Earth. According to Peto’s paradox, they should possess one of the most complex cancer prevention mechanisms. The minimal occurrence of oncogenesis in conjunction with such a long lifespan, however, is a mystery.
A team of scientists at the US National Institutes on Aging studied the accuracy and efficiency of DNA double strand break repair in bowhead whales, a process that astonishingly explained the long and cancer-free lifespan of the species. The team began by testing p53 activity in transfected mouse, cow, human, and bowhead fibroblasts. To their surprise, activity of the gene was lowest in the bowhead cells. From that, they were able to rule out apoptosis as a primary cancer resistance mechanism in the species. The researchers then created fibroblast cell lines of each species with knockout tumor suppressor genes and, surprisingly, found that the bowhead knockout cells needed only two mutations to become cancerous. This means that the species does not target cells after they experience cancerous transformation — rather, the cell has a mechanism that prevents the transformation from occurring at all. Additionally, the bowhead strain seemed to be most resistant to senescence (deterioration), which in conjunction with telomere shortening, describes aging at the cellular level.
The collected data demonstrates that the bowhead species is exceptionally evolved to avoid both cancer and accelerated characteristics of aging, but now scientists must ask how we may apply this new information to human oncology. The same team at the US National Institutes on Aging measured and compared DNA repair proteins in the whale using multiple assays and found that there was an abundance of cold-inducible RNA-binding protein. This protein, in conjunction with its partner PARP1, is a major player in the non-homologous end joining DNA repair pathway. Overexpressing the proteins in human fibroblast lines increases DNA repair efficiency and improves genomic stability overall.
“The pathways are certainly complex and intertwined, but the correlation between DNA repair and the prevention of death by cancer in the bowhead species is undeniable.”
Peto’s paradox accurately describes a general pattern in an evolved response to the threat of cancerous mutations. The theory doesn’t explain the unique mechanisms of the bowhead species though, perhaps suggesting a shift in the dialogue of cancer research. The pathways explored by cancer and aging scientists are certainly complex and intertwined, but the correlation between DNA repair and the prevention of death by cancer in the bowhead species is undeniable. The next step? Finding a way to increase the efficiency and accuracy of non-homologous end joining in humans to treat cancer before it even happens.