As of 2021, 367 million tons of plastics were produced globally, with over 22% composed of single-use items. Thanks to its durability, versatility, and ease of production, polyethylene terephthalate (PET) has become the most common form of plastic in disposable products like water bottles and grocery bags. Despite some successes in reducing single-use plastics in daily life, there is a fundamental caveat with the current strategy.
Plastic resists chemical breakdown due to its unique structure. Its synthetic, tightly ester-bonded hydrocarbon chains cannot be produced in nature. As such, common decomposers cannot break the linkages. Recycling is the primary tool to address plastic waste, however, only 9% of all plastic waste is recycled as it is a prohibitively costly and energy-intensive process. It is simply cheaper to manufacture more plastic. This has spurned researchers to search for alternative management tools.
Machine learning and rational design created…a variant with over 300-fold activity increase
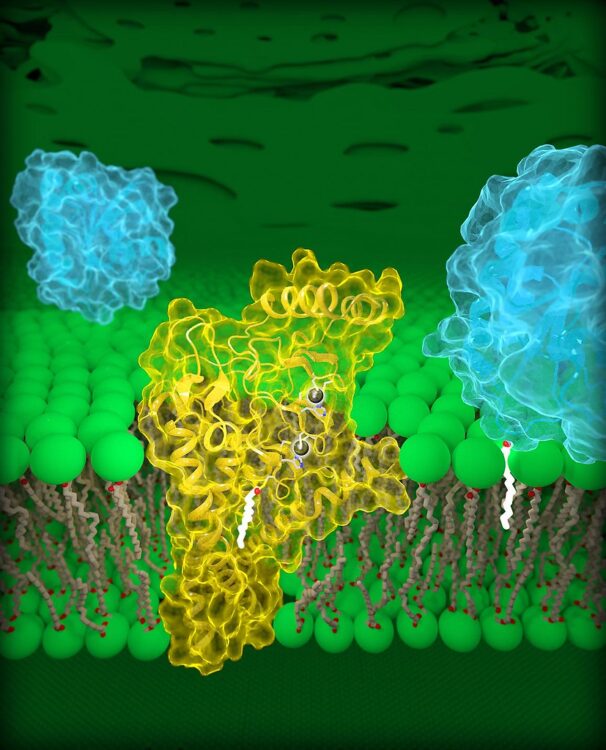
In 2016, researchers from the Kyoto Institute of Technology discovered Ideonella sakaiensis, a bacteria that produces the enzymes PETase and MHETase to break down PET into its monomers MHET, terephthalic acid (TPA), and ethylene glycol (EG), which it subsequently consumes. Although other plastic-eating organisms were isolated, wild-type PETase and MHETase have 5.5‐ to 120‐fold greater degradative abilities than other homologous enzymes and can function at a range of temperatures from room temperature to 65 degrees Celsius. This organism, which evolved to thrive on a completely foreign material in less than half a century, provides a promising mechanism for biochemical recycling.
Despite its intrinsic specificity to PET, without modification, the enzymes cannot break PET down fast enough to be applied in an industrial context. A colony of I. sakaiensis would need six weeks to completely degrade a low-grade plastic water bottle. However, researchers have employed a multitude of bioengineering techniques to enhance their catalytic activity and structural specificity to PET.
How do they function, and can they be improved? PETase’s region known as the binding pocket latches on to a long PET chain and cleaves the bond between MHET monomers, where MHETase then binds and cleaves into TPA and EG, which are non-toxic and can be reprocessed into new plastics. The physical shape of the enzyme determines the efficiency and speed of such a reaction – the more tightly the binding pocket can wrap around and bind to PET, the faster the enzyme can degrade its substrate.
Researchers have applied two types of protein engineering to this problem – directed evolution and rational design. The first relies on accelerating the evolutionary process with random enzyme mutations and screening the most successful conformations. Rational design refers to directly altering amino acids at critical sites to change the final configuration. These techniques have produced significant improvements -one group’s directed protein evolution mutant PETase had a 14-fold increase in PET degradation activity at 40 degrees Celsius. Another group applied machine learning and rational design to produce a variant with 31 degrees Celsius increase in melting temperature and over 300-fold activity increase under mild conditions.
Nature and bioengineering have produced a remarkable new system, but can it be scaled to a commercially viable solution?
Nature, enhanced with bioengineering, has produced a remarkable new system, but can enzyme-driven biorecycling be scaled to a commercially viable solution? A group at the National Renewable Energy Lab proposed a model for such a bioreactor network — a facility processing at a scale of 150 metric tons of treated PET would enter the enzymatic “bioreactor,” and be distilled into TPA and EG to be conventionally recycled. Various studies with the most recent PETase variants have projected 90% PET degradation extent at a purity of greater than 98%, less cost, and greenhouse gas emissions of virgin TPA. Despite such a promising theoretical yield, the initial cost of such a plant would be over seven times that of a conventional plant.
At such an experimental stage with a similarly high cost and no proof of concept, large-scale enzyme bioreactors may not start chewing up plastics in the next few years. However, more long-term models indicate that over five to 10 years (a typical recycling plant’s breaking even point), the high-quality materials, and sustainability may compensate in the long run. Overdependence on plastics and fossil-fuel consumption has had drastic consequences. Biorecycling may not be a silver bullet solution, however, with more investment at the intersection of protein engineering research and society-wide policy, the world has a platform for a promising bioeconomy and plastic-less future to strive towards.
Image courtesy of Flickr