Over 2 million new cancer cases are projected to be identified in the United States during 2024. That means over 2 million families are forced to rethink the upcoming years of their lives. Cancer has long been a topic of heartbreak and dread across the globe. Common treatments include surgery, chemotherapy, and radiation, but it isn’t uncommon for patients to exhaust their options. Luckily, antisense technology has emerged in recent years as a promising addition to cancer therapies, providing a method of reducing tumor size when it seems nothing else is working.
Each cancer case is a testament to how even the smallest DNA mutations can result in tragedy. Accumulated mutations in tumor suppressor genes and oncogenes, both of which regulate cell proliferation and the cell cycle, result in the uncontrolled cell division and metastasis into surrounding tissues that defines cancer. One such oncogene is ERBB2, which encodes the protein HER2 involved in mediating cell division and development. HER2 levels are often elevated in cancer cells due to mutations that dictate its overproduction, contributing to uncontrolled growth.
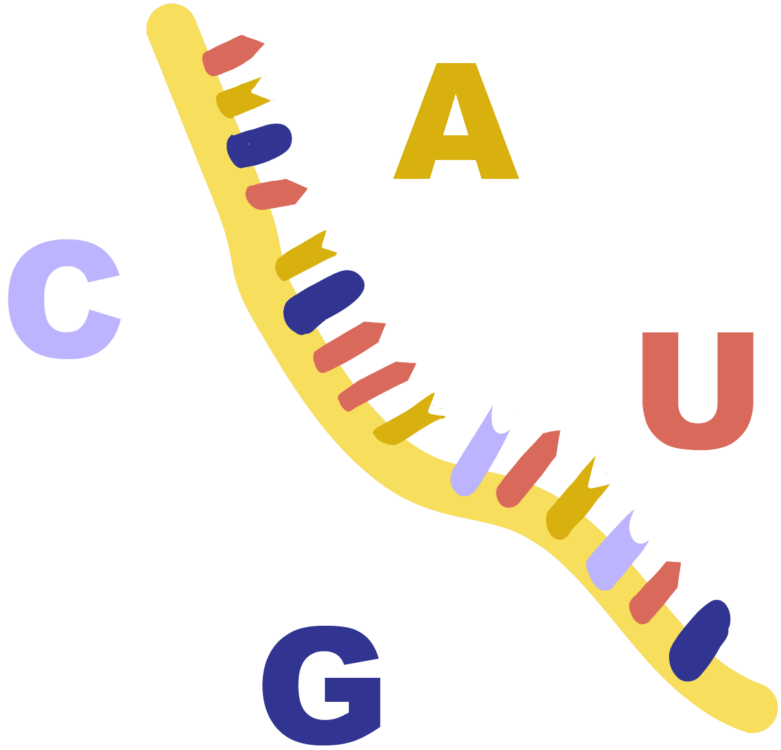
Antisense oligonucleotides (ASONs) provide a way to attack specific mRNAs and prevent translation of cancerous genes. ASONs are short, synthetic genetic strands that are specifically encoded to be complementary to a target mRNA and form an ASON-mRNA duplex. This prevents translation of the mRNA transcript into protein, leading to its disuse and degradation. Over time, ASONs can decrease the expression and action of a protein altogether. For example, preventing the translation of the ERBB2 mRNA can lower HER2 levels and restore regulation of the cell cycle, thus decreasing tumor mass. Most importantly, ASONs do this without many of the unfortunate side effects of chemotherapy or radiation.
“ASONs do this without many of the unfortunate side effects of chemotherapy or radiation.”
The first ASONs to be tested were linear and made purely of common DNA or RNA nucleotides. However, their half-life of fewer than 30 minutes in physiological conditions limited their therapeutic potential, as they degraded too quickly to have an impact. To slow the action of nuclease enzymes breaking down ASONs, chemical modifications were made to the molecules comprising each nucleotide. These modifications ranged from adding fluorine atoms onto sugars to bonding carbon chains to phosphate groups. The development of special ASON conformations and chemical configurations now extends the half-life of an ASON to as long as seven hours!
In 2022, Zhe Zhang, an affiliate of the China Medical University, and his team developed a prodrug-type ASON that was dumbbell-shaped with responsive disulfide (S–S bond) “switches.” By including a loop at each end of the oligonucleotide to create its namesake shape, it became more difficult for exonucleases to degrade the dumbbell-shaped ASON (DS-ASON), allowing it to act in the body for longer. The disulfide bonds keeping the DS-ASON together are stable extracellularly but prone to rapid breakdown in the tumor microenvironment. Zhang and his team used this to their advantage to “code” a time at which the DS-ASON will act; as soon as the prodrug enters the cytoplasm of a cancer cell, the disulfide “switch” is flipped to break the dumbbell conformation and release a linear active ASON. Quickly, the ASON binds to targeted mRNA and prevents the creation of that protein. To test this action, Zhang and his team targeted the gene RRM2 encoding the smaller ribosomal subunit, preventing the creation of this subunit and thus, the translation of many genes in the cancer cell. This eventually led to apoptosis and an antitumor effect.
Synthesizing the DS-ASON required manipulation of the conventional method of synthesizing oligonucleotides: the phosphoramidite method. This method uses solid-phase synthesis to polymerize oligonucleotides on a controlled pore glass surface. Unlike biological DNA or RNA synthesis, this chemical synthesis relies on various manmade protecting groups to prevent nucleotides from connecting in unwanted ways. Zhang and his team used modified dithiol phosphoramidites to introduce disulfide bridges into the oligonucleotide. This allowed the DS-ASON to self-assemble into the dumbbell-shaped conformation via reverse folding and annealing of complementary base pairs at the ends of the disulfide bridges to the main body of the oligonucleotide.
“The creation of a DS-ASON that had longevity in physiological conditions and exceptional antitumor performance opened doors for the development of future oligonucleotides.”
So, what does this mean for medicine? The creation of a DS-ASON that had longevity in physiological conditions and exceptional antitumor performance opened doors for the development of future oligonucleotides. In mice, an optimal DS-ASON significantly reduced tumor size by 78%. After refinement and further experimentation, these numbers could soar. Enlisting this protection strategy using ASONs could increase the speed and efficacy of RNA therapeutics as a whole, paving the way toward a world where cancer is a hurdle more easily jumped. While the technique needs further study, this type of molecular weight lifting will help cancer patients shed the weight of tumors, one oligonucleotide at a time.