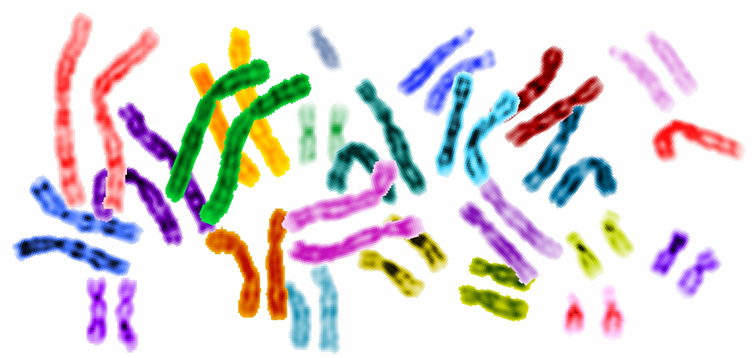
Spanning thirteen years, six countries, and hundreds of experts, the completion of the Human Genome Project marked one of the greatest scientific feats in the past century. The landmark scientific effort comprehensively sequenced 3.2 billion nucleotide base pairs and accounted for 92% of the human genome by the time the project ended in 2003. Today, almost 20 years later, scientists now revisit the project asking: How can we account for the remaining 8%?
This question led to the formation of the group “Telomere to Telomere” whose goal is to fill the “black hole” of the human genome project by sequencing telomeres and centromeres; these components of the human chromosomes were unable to be sequenced due to technological limitations during the original Human Genome Project. Now, the group has access to two major advancements in technology: The Oxford Nanopore DNA sequencing method and the PacBio HiFO DNA sequencing method. The Oxford Nanopore method can sequence up to 1 million DNA nucleotides in one reading, while the PacBio HiFi DNA method can read up to 20,000 nucleotides with almost perfect accuracy. During the original Human Genome Project, researchers were only able to sequence short pairs at a time, rendering the methods at the time costly and time-consuming.
Telomeres, which are repetitive sequences that protect and preserve the chromosome from nucleolytic degradation, were originally regarded as “junk DNA” by scientists. However, recent studies have found that telomeres house vital genes that influence protein development, chromosome division during meiosis, and those that define the human species. Telomeres have also been linked to various forms of cancer and aging, opening a new portal of potential diagnoses forms and diagnostics:
Telomeres, which are repetitive sequences that protect and preserve the chromosome from nucleolytic degradation, were originally regarded as ‘junk DNA’ by scientists.
“We’ve discovered millions of genetic variants that were previously not known across samples of thousands of individuals whose genomes have already been sequenced,” said Rajiv McCoy, an assistant professor at Johns Hopkins University. “We will have to wait until future work to learn more about their associations with disease, but a big focus of work now will be on trying to discover new genetic variations that were previously uncharacterized.”
In addition to telomeres, centromeres, which bind the two chromatids of a chromosome together also have the potential to give new insight into molecular genetic pathology. Centromeres are highly influential in neurodegenerative diseases and brain development and are speculated to be involved in immunodeficiency; centromeric region instability; and facial anomalies syndrome, which is a rare disease that is characterized by rearrangements in proximity to centromeres on chromosomes 1 and 16.
In addition to sequencing telomeres and centrosomes, the next step computational biologists are looking at is to sequence both maternal and paternal sets of chromosomes. During fertilization and meiosis, the blastocyst inherits one set of chromosomes from the mother and the other set from the father. The original project found it difficult to separate and match maternal and paternal sequences. The issue is described as having two puzzles in the same box: scientists have the sequences, but connecting and identifying if a sequence belongs on one chromosome or another remains a daunting yet rewarding task.
The issue is described as having two puzzles in the same box.
Since 1990, the Human Genome Project has accelerated medical research in numerous ways, from providing diagnoses at a molecular level, to opening doors to genetic engineering, to prevent these diagnoses. The study has also influenced our treatment of rare disorders. Now, there is increased cohesion of genomic profiles across multiple individuals in a population, helping identify correlations and other factors which may influence diseases. Although the project has been extended for over 30 years, the continuous progression of science has saved many lives and has the potential to save even more.
“This is the joy of science and research: the work is never complete,” said Eric D. Green, the director of the National Human Genome Research Institute. “Each advance opens new vistas of opportunities with the frequent sense that the best is still yet to come.”
Image courtesy of Wikimedia Commons