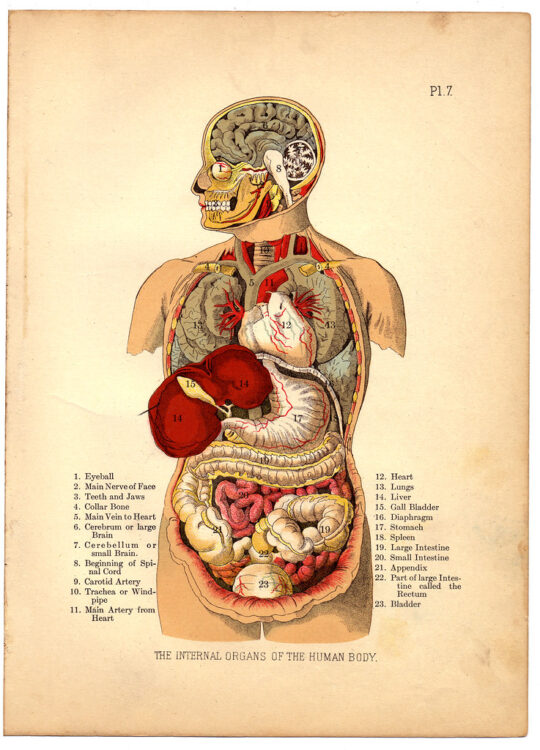
A patient desperately needs a new heart. They might wait several months for a donor organ, which may not be available before their condition worsens. Even if one is received, the body might still reject the transplant and work against the foreign cells introduced. Living with a transplanted organ means taking numerous drugs to prevent this immune response, often with a decreased quality of life or a shorter life span.
But what if it didn’t have to be this way? What if, given a small sample of a patient’s cells, a fully functioning heart composed entirely of their cells could be grown in a few months? While sounding like science fiction, generating functional organs like a heart is now a hot topic in biomedical research. In particular, 3D bioprinting seeks to apply technologies developed for 3D manufacturing toward printing functional organs and tissue constructs.
Traditional plastic-based 3D printing relies on a method called fused deposition modeling (FDM). In FDM, a small plastic filament is melted and deposited in a predetermined pattern. As it is deposited, the plastic hardens, and, layer by layer, a 3D object is built. This method has seen widespread success, creating strong and detailed parts with very little cost and manufacturing time. However, biological materials often do not behave like plastic. Cells and proteins can’t be melted and reformed like plastics and will collapse on themselves if printed using FDM methods. This limits the complexity of bioprinted constructs to near-2D geometries, a far cry from the complexity needed for an implantable organ replacement.
Since the materials used for bioprinting cannot be changed without compromising their biological function, alternative solutions are needed. One new approach is freeform reversible embedding of suspended hydrogels (FRESH), a new method for printing biological materials where — rather than changing the material that is printed — the surroundings are changed.
As detailed in a 2015 Science Advances study, FRESH is a technique that involves printing biological materials into a support material rather than into the air. This support material is composed of microscopic spheres of gelatin tightly compacted in a liquid medium. When small forces are applied, the support acts as a solid, allowing it to hold the printed constructs in place without deforming. When larger forces are applied, the material acts as a liquid, allowing penetration with a syringe for depositing print material. The print and support materials are carefully chosen so that the printed material will harden when it contacts the support. The support can then be melted and washed away, leaving just the print behind.
Researchers used this technique to 3D print a collagen heart valve, successfully replicating the structure found in an adult human heart. Remarkably, when exposed to pressure conditions mimicking the pumping motion of the heart, this valve opened and closed in a manner consistent with biological function over numerous cycles. Heart valve replacement surgery is a common procedure, usually performed with a valve from a pig, cow, or donor human heart. The prospect of an artificial valve substitute is extremely exciting as it can drastically reduce costs and increase the effectiveness of these procedures.
To further demonstrate the capabilities of FRESH printing of organs, researchers proceeded to print a full-scale model of a neonatal human heart out of collagen. This construct was created from an MRI model and accurately replicated the complex structures in the heart. Although a nonfunctional model, this construct is an amazing example of what this technology is capable of and an important step towards replicating the structure and function of human organs.
While valve and heart models are impressive, they are still only static components, lacking the movement and responsiveness present in human tissue. One of the most impressive applications of FRESH printing involves printing cell-laden ink into 3D tissue structures. Using this method, researchers created a model ventricle of human cardiac muscle cells and a collagen support structure. Over time, this ventricle exhibited synchronized contractions consistent with normal cardiac function, something that seemed impossible to replicate just a couple of decades ago. Although we may be a long way from replacing donor organs entirely, FRESH bioprinting shows great strides toward a printed-organ future.
Sources:
Science (2019). DOI: 10.1126/science.aav9051
Science Advances (2015). DOI:10.1126/sciadv.1500758
Image courtesy of Flickr