Conformational diversity and beauty in proteins
By Brian Cho, Chemical Engineering, 2023
Proteins account for 50 percent of the dry mass of most cells and form the machinery that allows cells to live. Why are they so abundant and how do their structures affect their functions?
Proteins are large, complex structures formed from tiny building blocks called amino acids, which are organic molecules containing both amino and carboxyl (acid) groups. Each protein contains a specific number of amino acids in a unique order. Intermolecular forces of attraction mold and bend the chain of amino acids as the protein exits its source, giving it its characteristic shape. Once fully folded, the protein quickly begins its job within the cell.
It is said form determines function. On a larger scale, honeycombs are shaped like stacked hexagons to reduce the space between “cells” of honey, increasing the amount the hives can store. This idea also applies heavily to protein function. Protein conformation only allows specific substrates to bind and interact with the protein itself. Molecules enter an active site where the protein then interacts with the substrate to modify it, proceeding to create and release a product into the surrounding area.
A great example of how protein structure affects function can be shown by carbonic anhydrase, the fastest protein in the world. A seemingly simple protein, carbonic anhydrase uses its amino acid side chains to rapidly turn water and carbon dioxide into carbonic acid and protons, increasing the acidification of the blood. In most parts of the body, carbonic anhydrase converts gaseous carbon dioxide into soluble bicarbonate. Once in the lungs, it’s converted back into carbon dioxide to be released into the air by breathing out. A simple mutation in any amino acid that contacts the water could lead to a loss of this function, slowing down the ability of organisms to breathe properly.
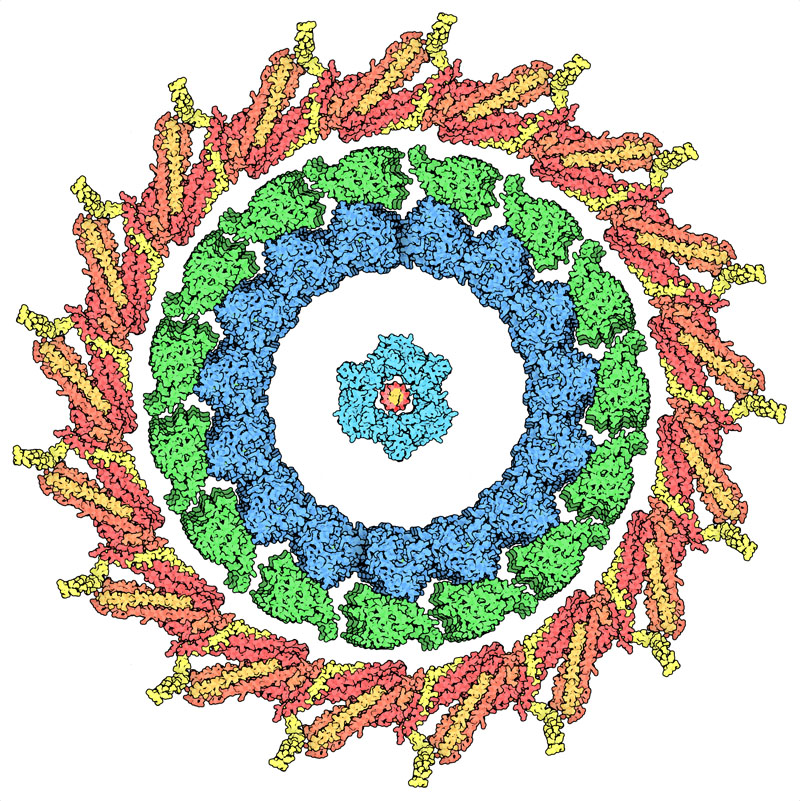
Although structure determines function, there are still beautiful proteins left to explore.
Although structure determines function, there are still beautiful proteins left to explore. This molecular structure represents the kinetochore ring surrounding a kinesin-microtubule complex and PCNA surrounding DNA. Clearly, there is a symmetrical feature to this protein complex. It is quite interesting to think about how these proteins form universally present shapes, such as embedded circles, to complete their functions.
As shown, proteins can take all shapes and sizes but one property will always remain universal: their structures allow them to function. Proteins work to keep cells alive by creating molecules, destroying waste, and even signalling messages. Their shapes keep us running, in a beautiful way.