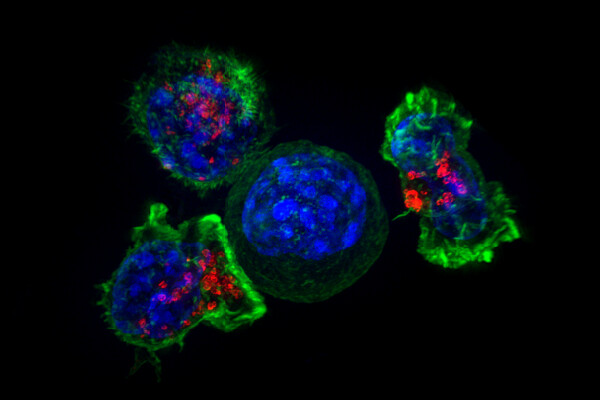
Cancer has been around for as long as humans have. The oldest known case of cancer comes from a bone dating back 1.7 million years ago from South Africa. Its earliest written record comes from ancient Egyptian physician, Imhotep, in which he characterizes an incurable malady that was “bulging and spread all over the breast.”
While solid tumors, like breast cancer, are removable with surgery, blood cancers constantly move around the body, making it impossible to isolate. Blood cancers also target the immune system, leaving the body with no protection and vulnerable to other infections. Chemotherapy, the use of chemicals to treat disease, is good at killing cells but not good at distinguishing between healthy cells and cancerous cells.
While solid tumors, like breast cancer, are removable with surgery, blood cancers constantly move around the body, making it impossible to isolate.
But could scientists use human cells as a weapon against cancer? William B. Coley from the Bone Tumor Service at Memorial Hospital in New York is regarded as the father of cancer immunotherapy research. He found that by harnessing the immune system’s natural processes, cancers could be forced into remission. In an experiment, he injected a weakened strain of Streptococcus pyogenes into patients’ tumors and activated an immune response, thus achieving durable remissions in a wide variety of cancers. One of the fastest emerging and most promising subsects of immunotherapy is a concept that builds off of Coley’s. Cells from the patient’s immune system are extracted and hijacked to target and kill cancer cells. One of these treatments, chimeric antigen receptor (CAR) T-cell therapy, has shown great promise, particularly in blood cancer.
Cells from the patient’s immune system are extracted and hijacked to target and kill cancer cells.
First-generation CAR T-cells could not persist in the body because none of the identifying molecules that were essential for eliciting an immune response from the T-cells were present. Without them, the first generation was ineffective. The second generation — created in 2002 — consists of two parts: the T-cell receptor component and the antibody component. The T-cell component can signal heavily for neutrophil production, which creates specialized white blood cells that kill cancer. The antibody component helps target the cancer cells by latching onto the identifying proteins found on the cancer.
The procedure for getting CAR T-cell therapy is extensive. First, patients have their blood drawn, in which only the white blood cells are collected. These cells are then sent to the lab, where they are engineered to target specific tumor cells. The patient must undergo a round of chemotherapy to cleanse their body of their old T-cells. The patient further receives infusions with the new T-cells, which now attack and kill the cancer cells.
In 2014, the FDA named CAR T-cell therapy a “breakthrough therapy” in treating cancers. After several years of research, in 2017, two CAR T-cell therapies were approved: one for acute lymphoblastic leukemia and another for advanced large B-cell lymphomas in adults. Although success rates are high and a cure seems promising, multiple side effects need to be monitored.
Despite its problems with toxicity and its relative newness, up to 90 percent of people who underwent CAR T-cell therapy are in remission.
CAR T-cell therapy can induce excess production of inflammatory molecules called cytokines. This can lead to a potentially fatal side effect called cytokine-release syndrome. Sometimes the symptoms are flu-like. Other times, symptoms include hypoxia (oxygen deficiency), capillary leakage (leaking of the smaller blood vessels), multiple organ failure, and neurological toxicities (including hallucinations, seizures, and confusion). Macrophage activation syndrome can also occur in people with autoimmune disorders, leading to over-multiplication of T-cells and macrophages, which — although treatable with antibodies — cause a great amount of pain and discomfort for patients.
Despite its problems with toxicity and its relative newness, up to 90 percent of people who underwent CAR T-cell therapy are in remission. Relapses have been shown to occur, but with further research, CAR T-Cell therapy can become a promising cancer treatment. Although only two treatments are currently approved, researchers have started to study CAR T-cell therapy with other cancers like multiple myeloma and chronic lymphocytic leukemia.
Without a doubt, these therapies have revolutionized the cancer battlefield. The puzzle is not impossible, but scientists continue to remain headstrong.
New England Journal of Medicine (1985). DOI: 10.1056/NEJM198512053132327
Science Translational Medicine (2013). DOI: 10.1126/scitranslmed.3005930