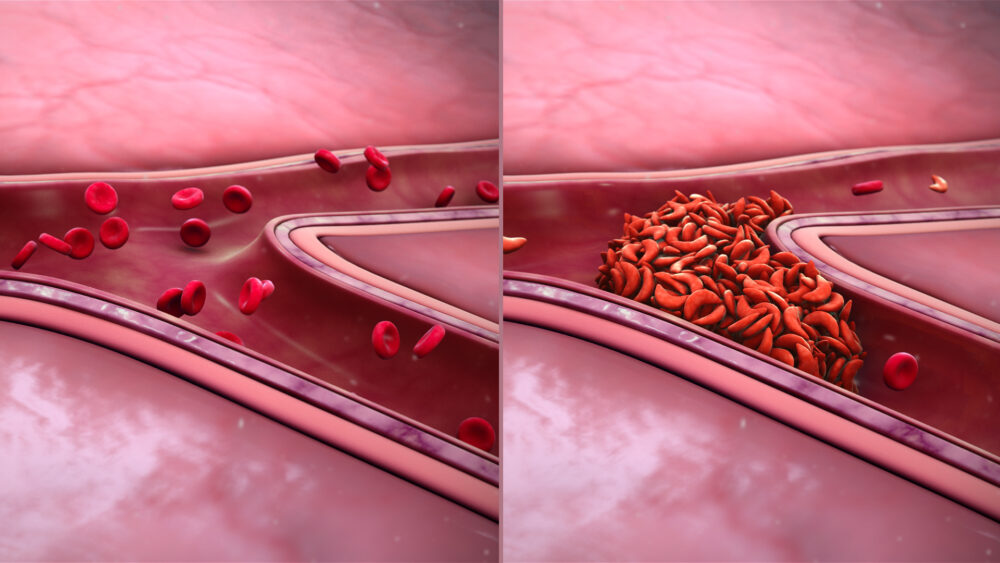
It has cost millions of dollars in research and made appearances in countless books and movies over the past 100 years. Sickle cell disease (SCD), first described in 1921, is a genetic condition with over 300,000 people born with it every year worldwide. The disease is caused by a mutation in hemoglobin, or proteins in red blood cells that carry oxygen to different parts of the body. People with SCD have hemoglobin that stick together and polymerize, creating long strands in red blood cells that morph into the sickle shape the disease is named for. Sticky hemoglobin doesn’t just cause differently-shaped cells, though; the red blood cells don’t properly distribute oxygen across the body, leading to frequent hospitalization, blood transfusions, and often premature death.
Existing treatments help some patients, but they are often ineffective, expensive, and fail to address the root of the problem: the mutated and inefficient hemoglobin. This is where CRISPR comes in. A relatively new technology for editing DNA, CRISPR-based therapies and medicines have the potential to revolutionize treatment for diseases that previously had few treatment options. By editing DNA, there are many different ways to potentially cure someone of the disease and restore their red blood cell functionality.
In 2018, CRISPR Therapeutics and Vertex Pharmaceuticals, two biotech companies located in the Boston area, began a clinical trial targeting patients with SCD. Their treatment works by tricking patients’ bodies into producing fetal hemoglobin, a certain type of hemoglobin that is typically only produced before birth. Scientists thought this would be easier to do rather than attempt to edit the mutation out of adult hemoglobin. In a 1974 paper published by The Journal of Clinical Investigation, researchers noticed that some patients with both the mutation for SCD and sickle-shaped red blood cells didn’t have the typical symptoms of the disease. They found that these patients’ fetal hemoglobin levels were much higher than most adults, meaning that their bodies never stopped producing fetal hemoglobin. While researchers didn’t know why at the time, they saw that these patients lived with few to no SCD complications, meaning that the fetal hemoglobin alone was delivering enough oxygen to keep them healthy.
When researchers looked deeper, they found that the gene BCL11A is responsible for turning off fetal hemoglobin expression post-birth. For BCL11A to be turned on, suppressing fetal hemoglobin, both its promoter and enhancer must be activated. People with these elevated fetal hemoglobin levels have a mutation in the BCL11A enhancer found exclusively in red blood cells, since every cell type has a different enhancer for the gene. This means that BCL11A never gets activated, and fetal hemoglobin continues to be produced into adulthood.
In nearly every way, they’ve been essentially cured of SCD.
This mutation is the basis of the 2018 clinical trial. The teams at CRISPR Therapeutics and Vertex Pharmaceuticals use CRISPR-Cas9-based gene-editing technology to destroy this enhancer, allowing patients to produce fetal hemoglobin and hopefully live as if they never even had SCD. More specifically, they extract stem cells from patients, filter out hematopoietic stem cells (blood cells before they’re differentiated), and edit these cells to destroy the BCL11A enhancer. While this editing process is ongoing, the patients are treated with chemotherapy to destroy some non-edited stem cells and weaken the immune system so it won’t attack the edited cells once they’re implanted. After ensuring that the edited cells are safe, doctors re-implant them into the patients.
The first results of this trial were released in June 2021 and were truly remarkable. Seven patients were treated, and all seven showed improvements. They had much higher levels of fetal hemoglobin, no longer required regular transfusions, and had no hospitalizations. In nearly every way, they’ve been essentially cured of SCD.
This treatment isn’t even the only one in development for SCD, though. In another high-profile trial, researchers at the University of California, San Francisco’s Benioff Children’s Hospital led by Dr. Mark Walters, a pediatric hematologist-oncologist, are working on a new method.
The first results of this trial were released in June 2021 and were truly remarkable. Seven patients were treated, and all seven showed improvements.
This treatment doesn’t focus on fetal hemoglobin; rather, it attempts to repair the mutation in the adult hemoglobin gene, which would see patients start producing normal, non-polymerized hemoglobin that no longer causes red blood cells to have a sickle shape. This study is scheduled to start on December 1 of this year.
CRISPR treatments may be new, but they’re already proving themselves to be some of the best tools we’ve ever known to treat genetic diseases, breathing new hope into places where there was little to none before.
The Journal of Clinical Investigation (1974). DOI: 10.1172/JCI10779
Science (2014). DOI: 10.1126/science.1242088