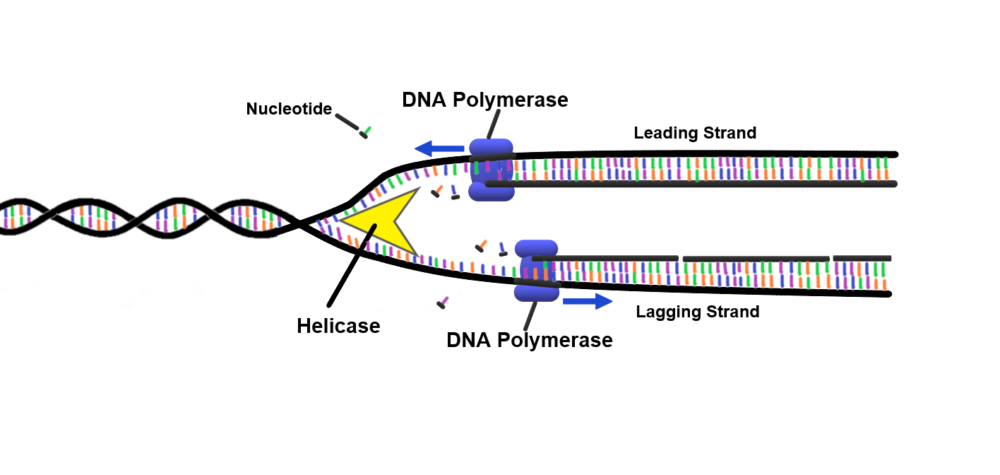
Life on Earth has been evolving, adapting, and refining itself for over three billion years, leading to the intricate organisms which roam the world today. After such a long time, one would think that cells would have developed ways to ensure high-fidelity during gene replication. After all, cells must replicate their genome to pass down the fruits of their evolutionary labors for the last few billion years. In fact, most cells have continuously purified the replication process, making errors at rates less than one base change per genetic division. For humans, this means an average of less than one error per billion bases replicated. However, replication rarely occurs in ideal conditions. Factors like UV-light exposure, environmental toxins, and the food you eat can cause the formation of DNA lesions, or damaged sections of DNA. Many of the lesions arrest high-fidelity replication enzymes like standard DNA polymerases, requiring other enzymes to repair DNA breaks and complete the recently understood process of translesion DNA synthesis.
Most cells have continuously purified the replication process, making errors at rates less than one base change per genetic division.
By learning the mechanism of translesion DNA synthesis, researchers might understand why this function has persisted through evolution. During DNA replication, a family of enzymes — called DNA polymerases — are responsible for synthesizing new DNA strands. However, these structures fail to replicate DNA with lesions. For the cell to survive and pass down its genetic information, it must use a different family of polymerases, aptly called translesion or “error-prone” polymerases. Though it sounds simple, nobody understood the mechanism behind the replication of these lesions until a 2001 study led by Haruo Ohmori identified the various families of polymerase. Since the genetic and structural makeup of standard polymerases and translesion polymerases were so similar, many researchers believed them to be the same, and their functional differences went unidentified. Once technological advancements were made, however, researchers found that polymerases responsible for traversing lesions had one major structural difference from normal polymerases: a larger active site, where the proteins bind new bases to the old DNA strand. This structural difference meant that these polymerases could bind to damaged DNA strands that the smaller active site could not. Larger active sites come with adverse side-effects, since the looser grip has a higher likelihood of misincorporation of bases when repairing lesions, inducing higher rates of mutations. However, mutations don’t truly matter in damaged DNA, because had it not been for the mutation, the DNA could not have replicated over the lesion, and the cell would have died anyway.
The danger comes when translesion polymerases are released upon undamaged DNA. Though rare, it has been observed that the structures responsible for regulation of translesion polymerases will allow them to encounter undamaged DNA. This is likely to occur in undamaged cells that are neighboring, for example, UV-light-induced damaged cells, according to a review led by Alison Rattray. Once in contact with undamaged DNA, translesion polymerases are free to replicate, causing more mutations than the average one per billion bases. Intuitively, more genetic mutations would be detrimental to life since it randomly changes billions of years of evolution. For the most part, this is true, since the expression of translesion polymerases in undamaged DNA is linked to failure to produce necessary gene protein products, cell death, and cancer formation.
The expression of translesion polymerases in undamaged DNA is linked to failure to produce necessary gene protein products, cell death, and cancer formation.
So why is it that organisms haven’t found a way to prevent something with such drastic consequences? With the advent of whole-genome sequencing, more and more translesion polymerases have been discovered, following the evolutionary trend that they increase biological fitness. Many single-celled organisms live in highly competitive environments, where they need every advantage they can get. For them, increased mutagenesis can mean accelerating evolution and getting an edge over competitors. In multicellular organisms, like humans, benefits can be seen in somatic hypermutation (SMH) induced by “error-prone” polymerases. SMH is the process of dramatically increased mutations in antibody regions, which helps strengthen the immune system and provide immunological diversity. For single and multicellular organisms, increased mutations can prove to be beneficial by either accelerating evolution or promoting genetic diversity.
Though error is naturally deemed a destructive term, “error-prone” polymerases are a unique case where mistakes can be favorable. Organisms must selectively use low-fidelity structures to balance the risks and rewards of allowing “error-prone” polymerases to interact with undamaged DNA. Our understanding of translesion synthesis has dramatically increased in recent years, but much is still unknown about translesion polymerases, and it is imperative to continue learning about their role in the evolution and genetic diversity.
Annual Review of Genetics (2003). DOI: 10.1146/annurev.genet.37.042203.132748
Cold Spring Harbor Perspectives in Biology (2013). DOI: 10.1101/cshperspect.a010363
Journal of Nucleic Acids (2010). DOI: 10.4061/2010/643857
Molecular Cell (2001). DOI: 10.1016/s1097-2765(01)00278-7