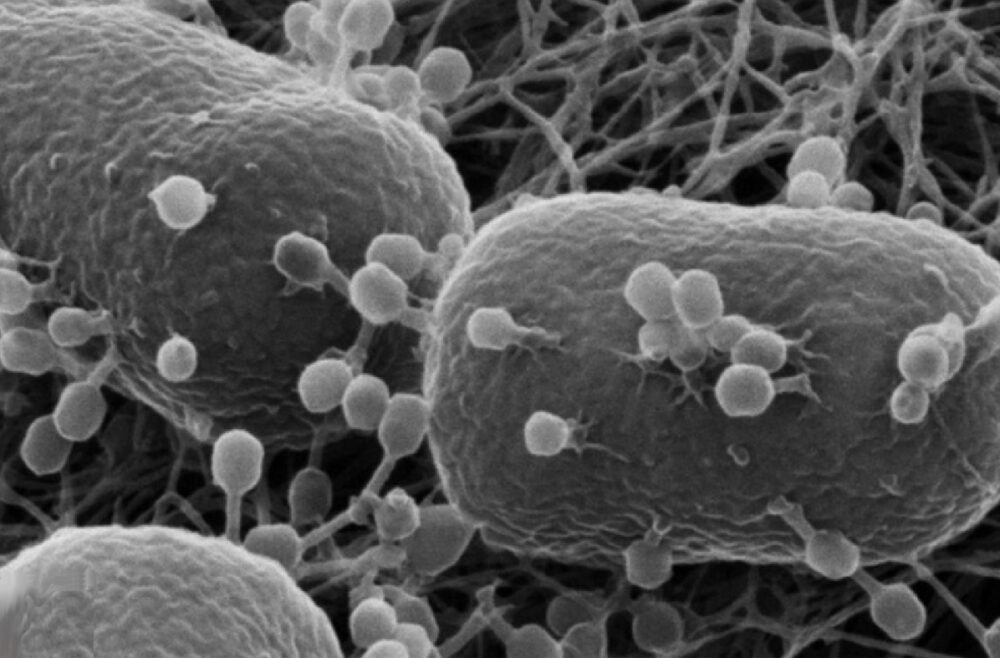
Viruses can be catastrophic. Their natural purpose is to infect and replicate, hijacking hosts regardless of the consequences. They can be deadly and are a large source of suffering, even in our modern society. Smallpox, influenza, and COVID-19 are clear examples of viruses that have had widespread, devastating public health effects. Therefore, it seems counterintuitive to consider using viruses for therapeutic purposes. However, one class of viruses that do not infect animal cells could prove useful as a delivery method for cancer drugs — bacteriophages.
Bacteriophages naturally only infect bacteria and are incapable of infecting eukaryotic cells. Once they recognize and attach to a bacterium, they insert their genetic information and either integrate their genome into that of the host, as in the case of lysogenic phages, or directly produce more phages and break apart the bacterium, as in the case of lytic phages. The key features of bacteriophages are their specificity for infection and ability to deliver genetic material, properties that make them well-suited for therapeutics.
Delivery is a constant struggle for chemotherapeutics. Poor delivery systems lead to inefficiency as a drug may spread to untargeted areas within the body and reach tumors at a concentration too low to be effective. A lack of specificity is also the primary cause of off-target effects, leading to the detrimental side effects often associated with chemotherapy such as nausea and hair loss.
“The key features of bacteriophages are their specificity for infection and ability to deliver genetic material, properties that make them well-suited for therapeutics.”
Through genetic engineering, phages that bind to cancer-specific target receptors can be produced. Unlike other viral vectors used in therapeutics, such as adenoviruses, phages are not pathogenic. This means that therapeutic phages can bypass healthy cells without infecting them, preventing off-target effects. This lack of interaction makes them less likely to elicit an immune response. An immune response toward the phage would cause sickness in the patient and reduce the effectiveness of future phage-based therapeutics, while also interrupting the desired effect of the therapy.
Once the phage reaches the tumor, it binds to its unique target cancer receptors and is taken into the cancer cells. From there, the genetic payload serves a variety of functions depending on the goal of the therapy. The payload can work indirectly as immunotherapy by incorporating genetic information leading to the production of membrane-bound signals in a process known as “phage display.” This signaling display acts as a beacon that allows for beneficial targeting by the patient’s immune system, allowing previously hidden or masked cancer cells to be identified and killed. A more direct bacteriophage-based cancer therapeutic is the delivery of genetic material that causes death in the target cell. Once brought into the cell, the phage would release a DNA or RNA payload that could produce or alter the expression of tumor-killing proteins. For example, a team used recombinant phage particles to deliver a long non-coding RNA that restored the expression of p53, an important tumor suppressor gene frequently mutated in cancer. This reintroduction of a key protein induced apoptosis and decreased cancer proliferation.
Alternatively, scientists can engineer phages to display cancer-specific antigens on their capsids, or outer coatings, making them effectively act as cancer vaccines. This would not require tissue-specific delivery as the foreign antigens would elicit an acute immune response toward the cancer, regardless of phage localization. That immune response would then trigger the production of antibodies targeting the tumor, marking it for attack. The phage-based vaccines induce a stronger response when compared to a similar DNA vaccine as they can be engineered for increased immunogenicity and specificity. Additionally, the stored adaptive immunity towards particular cancers provided by the vaccine approach is especially beneficial in the case of resurgence.
Bacteriophages represent a possible solution to one of the biggest roadblocks in drug therapies: delivery. Their structure and specificity give them a variety of advantages over previous generations of drug delivery. Phages are stable in the body and are adapted to bypass eukaryotic cells. Importantly, they are also cheap to produce and easily engineered, allowing for flexibility in function. This flexibility permits multiple avenues of research and a wide range of potential therapeutics. In the burgeoning era of highly personalized genetics-based oncology, phages could play a key role in making patient-specific medicine biologically effective and economically feasible.
“In the burgeoning era of highly personalized genetics-based oncology, phages could play a key role in making patient-specific medicine biologically effective and economically feasible.”
Image courtesy of Wikimedia Commons