The electron exists in a state of superposition: They inhabit multiple states simultaneously. For example, an electron can be in one quantum state as well as a different one. This doesn’t mean that it is in both states at once but that it is in a superposition of both states. It is both and none of them at the same time.
At first glance, this spectacle doesn’t seem to be all that interesting. A closer look and the greater implications slowly reveal themselves. In the fields of astrophysics, quantum computing, AI, and virtually every other scientific field, the study of electrons and their properties of duality can be applied.
“In the fields of astrophysics, quantum computing, AI, and virtually every other scientific field, the study of electrons and their properties of duality can be applied.”
This seemingly arbitrary fact of quantum mechanics diverges significantly from the intuitive laws of classical physics. Classical physics is the basis for describing all physical phenomena excluding quantum particles. Its limitations were discovered only a century ago with the advent of Max Planck’s famous proposition for the quantization of light — the idea that light exists in discrete units. This discovery paved the way for studying a new subset of the physical world.
Louis De Broglie’s 1924 paper in the Philosophical Magazine kicked off a new wave of scientific thinking. His declaration of the electron behaving as a wave was a major divergence from the elegant calculations of classical physics and a huge step forward in quantum mechanics. Then came Werner Heisenberg, who proposed the uncertainty principle, and Erwin Schrödinger who theorized a way to describe the physical motion of a particle in the quantum state. Heisenberg boldly attributed a wave-particle duality to electrons, which detailed how the electron acts as both a particle and a wave and cannot be separated from either identity.
In college chemistry, these technicalities of electron behavior are never discussed. For those interested in the physics side of chemistry, this basic knowledge of quantum mechanics is troubling. I can’t help but wonder what these quantum particles mean in the context of my basic chemistry classes.
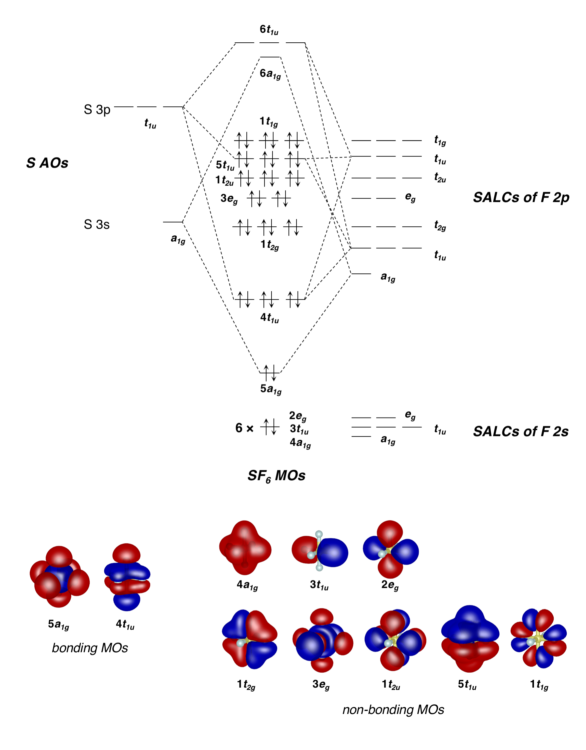
My main qualm is with molecular orbitals, which utilize the wavefunction in the form of Ѱ2 to approximate electron probability. This idea is used to represent orbitals using spherical shapes that recognize the locations of electrons within molecules. These orbitals are used to predict molecule stability, bond energies, and a multitude of other incredibly important chemistry concepts. Now, employing a quantum mechanical lens, the subjugation of electrons under the atoms it “belongs to” in a spherical cloud suddenly seems like a much squishier concept.
In my organic chemistry lectures, I find myself puzzled on how quantum mechanics might agree with or defy the class’s major concepts — the course curriculum glazes over important connections to quantum theory. For example, partial donation of electrons from one atom to another is closely related to superposition, but the concept is not taught in tandem with superposition. Further, the delocalization of electrons can be confusing. This term refers to the idea that electrons are not simply fixed between two atoms in a covalent bond but shared between multiple atoms and bonds. Yet, chemistry courses often leave out the underlying quantum mechanics explanations: How might the wavefunction predict this? In other words, if the position of a delocalized electron is measured, will the measurement reflect or contradict the position organic chemists might predict through the use of molecular orbital theories?
Within the field of quantum mechanics, there is a myriad of theories, philosophical discourse, a rich history of discovery, and an interesting evolution of scientific thinking. This has provided the world with a variety of plausible yet romantic speculations based on mathematical derivations and philosophical assumptions. Einstein gave us the possibility of hidden variables in quantum states, which proposed inconsistencies in quantum mechanics, and Hugh Everett gave us the many worlds interpretation, which rationalizes how every measurement of a particle in a state of superposition causes infinite instantaneous fragmentations of the universe. Now, we have quantum superposition in quantum computing and electron-based advancements in medicine.
The electron is first introduced to us as an inconsequential subatomic particle, but in reality, it has an infinite number of complex applications and its own set of governing rules separate from classical physics. It is perhaps the most curious and perplexing particle of the natural world, and I wonder if college chemistry courses will ever delve into the nuance of this concept, or more importantly, if modern science will ever be able to perfectly portray it.