Neurodegenerative diseases are characterized by rapid escalation of cell loss, a consequence of progressive decay of structure and function of neurons. Multiple system atrophy (MSA) is a rare neurodegenerative condition in which central nervous system function is degraded. Atrophy refers to the death of neuronal cells which can have dramatic effects on an individual’s function. There are two forms of MSA: the Parkinsonian type (MSA–P) and Cerebellar (MSA–C) type.
MSA–P presents as stiffness in mobility, coordination loss, and autonomic dysfunction which can be attributed to major cell loss in the brain’s striatonigral region, a hub for movement and balance. On the other hand, MSA–C is characterized by cell degeneration in the olivopontocerebellar region, causing balance issues, vocal deficits (speech and swallowing), and ocular irregularities. The onset of both forms of MSA typically occurs in adulthood, usually affecting individuals in their 50s. Upon diagnosis, medical experts estimate MSA leads to a shortened life expectancy within a decade but severe disability may occur at the halfway mark. The majority of MSA treatment research focuses on minimizing the progression of the disease by preventing irregular protein regulation, impaired synapses, and excessive cell death, which are widely considered the “hallmarks of neurodegenerative diseases.”
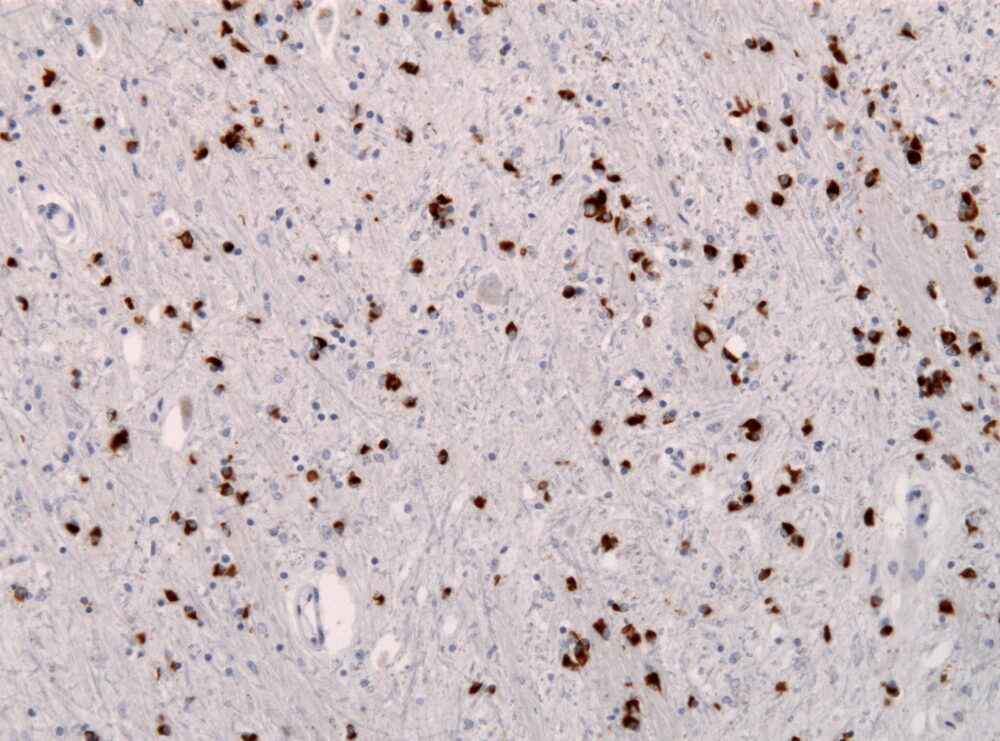
Research scientists have determined all patients with MSA experience the buildup of irregular proteins in glial cells, which provide neuronal support. Specifically, a protein called ɑ-synuclein (aSyn) is misfolded in those with MSA which causes disruptions in cell function, especially to the central nervous system. Large amounts of aSyn are found in glial cells responsible for supporting electrical signaling between neurons. In its properly folded form, aSyn is responsible for regulating neurotransmitter release into neural synapses. aSyn buildup is a commonality between MSA and Parkinson’s disease, also causing similar motor issues and also mental health issues. Both of these conditions fall under the umbrella term synucleinopathy, which refers to a group of neurodegenerative disorders that contain these ɑ-synuclein aggregates.
In May 2024, Tokyo Medical and Dental University (TMDU) investigated a form of treatment for Parkinson’s disease called antisense nucleic acid therapy, which could potentially apply to MSA since they are synucleinopathies. Antisense nucleic acid therapy involves the use of antisense oligonucleotides (ASOs) to prevent further neurodegeneration. ASOs bind to RNA, lowering transcription levels of the aSyn gene to prevent further buildup of aSyn. TMDU Professor Tetsuya Nagata explains that ASOs are advantageous because they ensure the existence of normal aSyn levels in the cell but decrease levels of the harmful version of the protein: ASOs “could offer higher safety and efficacy by both retaining the natural physiological functions of the protein while inhibiting the spread of pathogenic aSyn.”
Using mouse models, the TMDU researchers injected the misfolded form of aSyn into the striatum in either the right or left hemisphere to replicate aSyn buildup in synucleinopathies observed in MSA. They examined how misfolded aSyn spreads through the brain, and analyzed how injecting ASOs before or after the injection of misfolded aSyn impacted this spread pattern. ASO injections to the same site of misfolded aSyn injection caused a reduction in naturally occurring aSyn, which then led to a waning response in the spread of misfolded aSyn. These outcomes may indicate that by lowering normal–type aSyn, the spread of harmful aSyn can be mitigated which could lead to synucleinopathy treatment.
These new findings are exciting discoveries for the neuroscience research community. Still in the early stages, this research presents an opportunity to not only tackle treating MSA but other prevalent neurodegenerative disorders such as Alzheimer’s, Huntington’s, and Parkinson’s disease. In addition to ASOs, scientists are continuing to focus on learning more about early stage neurodegenerative disorders to shift the focus of treatments from management to prevention. As a rare and difficult disease to manage, studies like those conducted at TMDU have shown the potential to apply genetics in the treatment of MSA and other neurodegenerative diseases to help scientists better understand the condition.
“This research presents an opportunity to not only tackle treating MSA but other prevalent neurodegenerative disorders such as Alzheimer’s, Huntington’s, and Parkinson’s disease.”